FDA-Cleared SaMD by the Numbers

If industry buzz is any measure of actual activity, then Software as a Medical Device (SaMD) is the current darling of the MedTech world. Despite this, it’s difficult to quantify just how many SaMD have reached the market in the U.S. While the information needed to identify a medical device as SaMD is stored in the FDA’s public databases, there is no single data query (or multiple queries) that would return a complete list.
To gain a clearer picture of the state of SaMD approvals in the U.S., Orthogonal worked with Brian Binkowski, an experienced SaMD consultant, to build its own database. Using the definition of SaMD from the International Medical Device Regulatory Forum, we examined the FDA’s public databases to compile a running list of SaMD that now includes 483 individual devices cleared by the FDA and analyzed our current pool of data for insights on the current state of SaMD and overall trends. Here is what the data has to say.
FDA-Cleared SaMD by Year
Looking at the year-by-year data, SaMD clearance has generally trended upwards since the first product in our list was cleared in 1996. After two decades of clearances fluctuating between one and 11 per year, 2016 marked the start of an upward trend, with clearances nearly doubling year-over-year in 2020 and again in 2023.
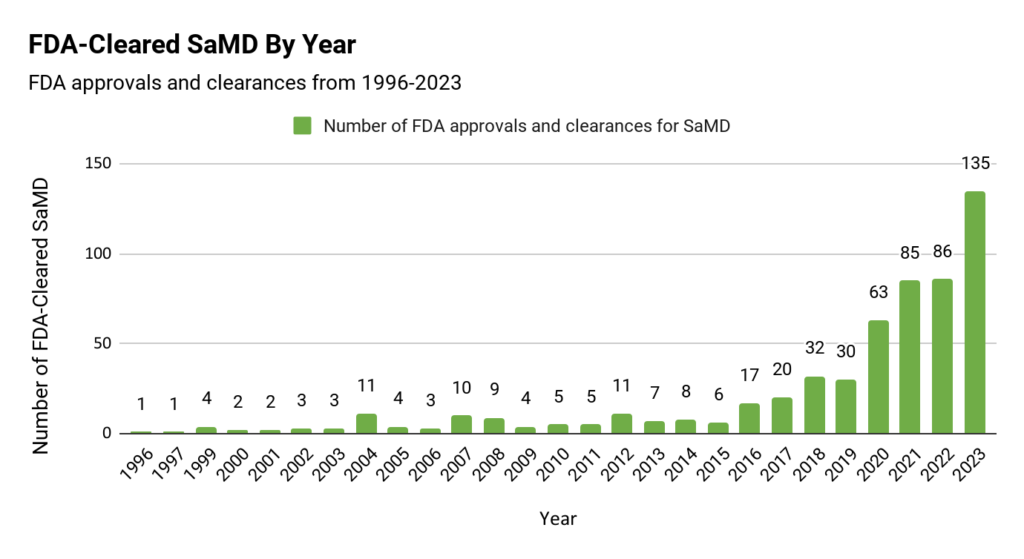
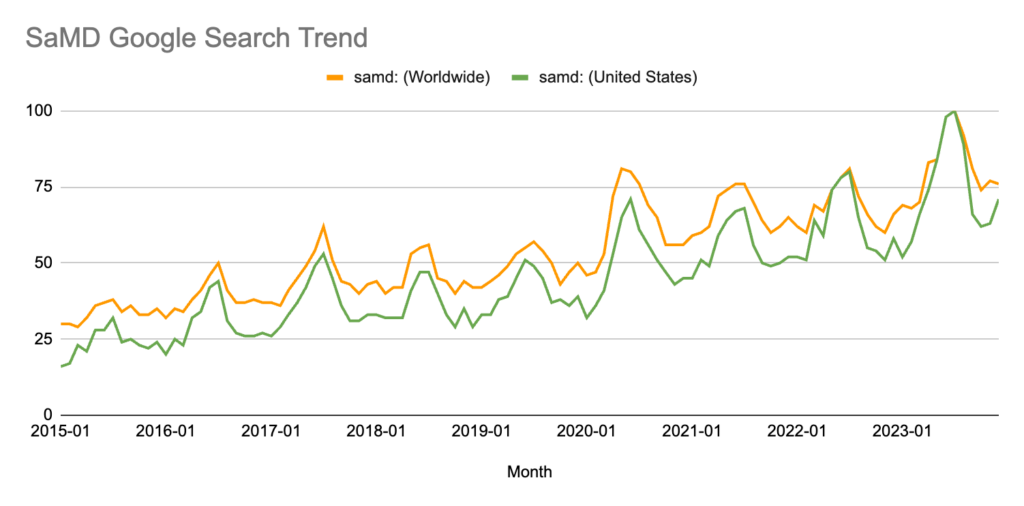
Interestingly, this graph has a visible correlation with research on Google Trends on the prevalence of searches for “SaMD” in the U.S. Search results show a continuous upward trend that hit an all-time peak in 2023. The increased number of SaMD products entering the market seems to correlate with the growing awareness of SaMD amongst the general U.S. population.
The jump observed in 2020 in our data made us wonder how many SaMD were given emergency use authorization (EUA) by the FDA during the COVID-19 pandemic. While many connected devices—i.e., physical device hardware with software embedded on the device or in a companion app loaded onto a users’ smartphone—were approved under EUA, we have identified only three pure SaMD products which have received EUA: ECG Low Ejection Fraction Tool (ELEFT), COViage and the CLEWICU System. The jump in FDA clearances since 2020 was likely impacted by the digitization of MedTech during and after COVID, but the pursuit of an EUA for SaMD during the pandemic was likely not a driving factor in raising this number.
U.S. vs. Non-U.S. Companies
We also looked at how many of the companies that received clearance for their SaMD in a given year were identified as being based in the U.S. and how many were based internationally. Interestingly, we see multiple years where the majority of FDA clearances belonged to companies registered outside of the U.S.—52.94% in 2017, 60% in 2018 and 51.39% in 2021.
Which non-U.S. country submitted the most SaMD? The Netherlands claimed the top spot on our list with 24 submissions, the UK and France tied for second with 23, Korea made third with 20, China and Canada tied for fourth at 19, and Germany claimed fifth with 17.
FDA-Cleared SaMD by Specialty
The FDA classifies medical devices into one of 19 medical specialty “panels.” The majority of SaMD fall into the radiology panel, comprising 64.3%. Second place goes to the cardiovascular panel, representing 12.9% of devices, and third to the general hospital panel, accounting for 4.4%.
FDA-Cleared SaMD by Company
What is the breakdown of SaMD clearances among the 308 unique companies on our list? The majority of companies (73.7%) have just one clearance. Companies with two clearances account for 14%, and companies with three clearances account for 6.2%.
Which company ranks the highest on SaMD clearances? Siemens Medical Solutions USA, the U.S. branch of Siemens Healthineers, has the most with 14 unique submissions. Behind them is Medis Medical Imaging Systems, B.V. with 10, Shanghai United Imaging Healthcare Co., Ltd with 9, and Pie Medical Imaging B.V. with 7.
Analyzing our draft list by the numbers highlights areas where our data aligns with wider industry trends and also reveals potential gaps in our data. For instance, we anticipate that the distribution of foreign countries will change as our list grows and we look forward to sharing updates on the state on SaMD clearance in the U.S. as this category continues to grow in health care.