Adhesives’ Crucial Role in Wearable Medical Device Design
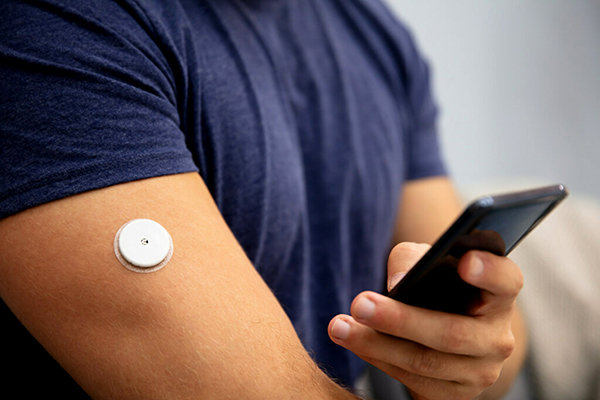
When it comes to developing wearable medical devices, design engineers need to consider many aspects, so it is not surprising that certain areas are not thought of or prioritized until later in the design process. One area that may be an afterthought are adhesives. As an essential material that creates an intimate connection between the device and its user and is paramount to functionality, the consideration of adhesive should be brought in at the beginning of the design process.
Creating a high-performing, stick-to-skin wearable medical device requires a secure, reliable hold for the desired duration of use. Without it, devices could prematurely fall apart or de-bond from skin, potentially rendering the device incapable of collecting the data users rely on to make critical health decisions. This is where adhesives come into play. However, not every adhesive is right for every application. And like most steps in the process, it requires several considerations to identify the right one for the application.
Before starting the design process, engineers should ask themselves the questions below to help them select the right adhesive at the beginning of the design process based on the intended use of the wearable device.
1. What wear time am I aiming for?
It is not uncommon to assume the strongest adhesive is always the ideal option because it will maximize wear time. But it is not always the best choice, especially if it is intended to stick the device to skin. Different types of adhesives adhere differently over time. If a device is only intended to be worn for a few days, an adhesive that’s at peak strength during removal can strip away the top layers of the skin. Ideally, longer-term wear devices, potentially worn up to two weeks, not only require a strong adhesive but also one that is gentle and breathable to help keep skin healthy.
2. What environmental conditions are at play?
Climate can affect how adhesives perform and change how skin acts. Adhesives that perform as intended in dry, mild climates may not exhibit the same properties in wet, humid climates. Humid climates can make skin dewier and produce more sweat than skin in arid climates. Considering what climate your device will be used in will aid in material choice.
3. What are the target end-users’ demographics?
Not every adhesive is compatible with skin at every stage of life. For example, patients with underlying medical conditions, newborn and elderly skin are more fragile so the selected adhesive must keep these sensitivities in mind. Full-term newborn skin can reach structural and functional development equal to an adult within the first year of life.1 Adolescent skin presents other obstacles, like increased oil and sweat production. Healthy young adult skin can be the easiest stage of skin to stick to because it is at its most durable and elastic. As skin continues to age, it loses hyaluronic acid, resulting in a stiffer, drier, less elastic and more fragile state. Knowing your end-user’s age and health conditions can help guide your adhesive selection process.
4. What will this adhesive stick to?
If the adhesive is sticking the device to skin, it needs to allow the skin to breathe, flex, move and expel moisture. Skin also grows hair and as discussed earlier, changes with age, making conformability and gentleness upon removal particularly important. In regard to the device itself, the goal of the adhesive is to keep parts from shifting or rubbing against each other, so strength and resistance to friction are the important characteristics.
5. Where on the body will the end-user wear the device?
As we discussed earlier, more sensitive skin can be less tolerant of devices covering it or adhesives sticking to it, so additional criteria must be considered to ensure the device operates as intended. It is important to consider how the thickness of the skin’s three layers—the epidermis, dermis and subcutis—varies across the body. Thinner and/or highly sensitive skin, like the eyelid, will require a different type of adhesive compared to the palms of hands or bottoms of feet.
6. What sterilization method makes sense for my device?
Sterilization can alter the properties of some materials. Considering which method to use on the front-end can help save designs from changing down the line. To mitigate risks, take the time to understand the three most common sterilization methods—ethylene oxide, e-beam and gamma radiation—and how each may affect the finished device. For example, ethylene oxide is a time-consuming process, but it can have a lower impact on materials versus gamma radiation which is fast-acting but may alter the performance of most adhesives.
7. Will this adhesive be compatible with my other materials?
Other materials being used in the device’s design will impact the adhesive selection, so it is crucial to do research ahead of time to determine the best compatibility. For instance, polyethylene materials are easy to work with and compatible with most adhesives but may require pre-treatment or priming to make a strong hold, whereas a polyester material is easy to adhere to but hard and inflexible. An experienced materials supplier should be able to discuss all available options to find the best-suited solution for the device’s intended use.
8. How will my design respond to the manufacturing process?
Not all adhesives are compatible with all manufacturing processes. Some adhesives are able to withstand the speed and friction of certain processes, while some are too soft and will gum up equipment. Consider working with a converting partner experienced with your materials to run experiments using a final device on a pilot or full-scale process equipment. It will help you understand how larger-scale production will go and help avoid redesigns, delays or cost overruns.
Asking these questions at the beginning of the design process versus the end will help you identify the best adhesive for the intended use and help create a high-functioning wearable medical device. As a result, costly missteps and errors can be avoided, saving time and money that can be used towards creating life-changing devices.
References
- Oranges, T., (2015). Skin physiology of the neonate and infant: clinical implications. Advances in Wound Care. Vol (4) 10: 595-604.